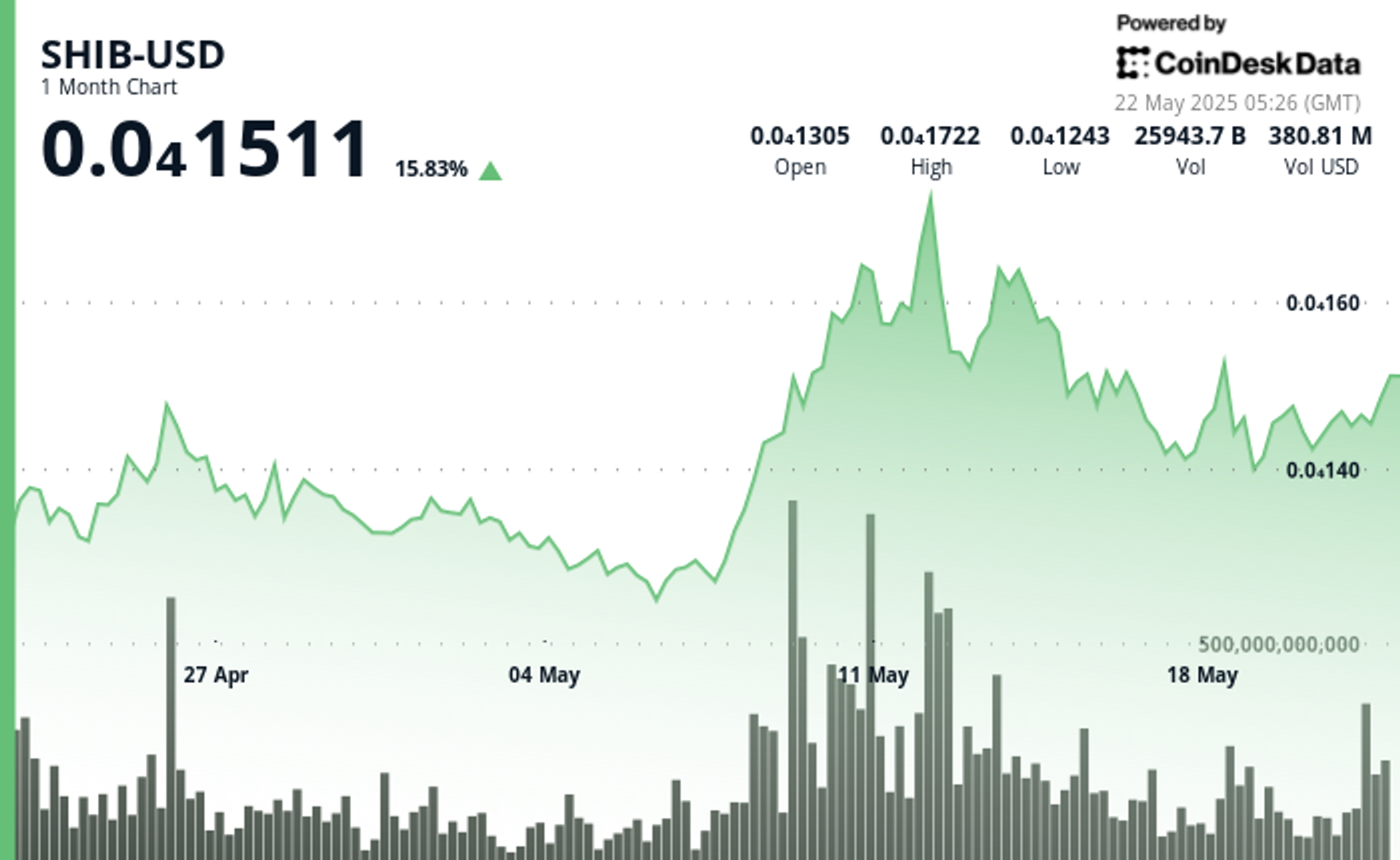
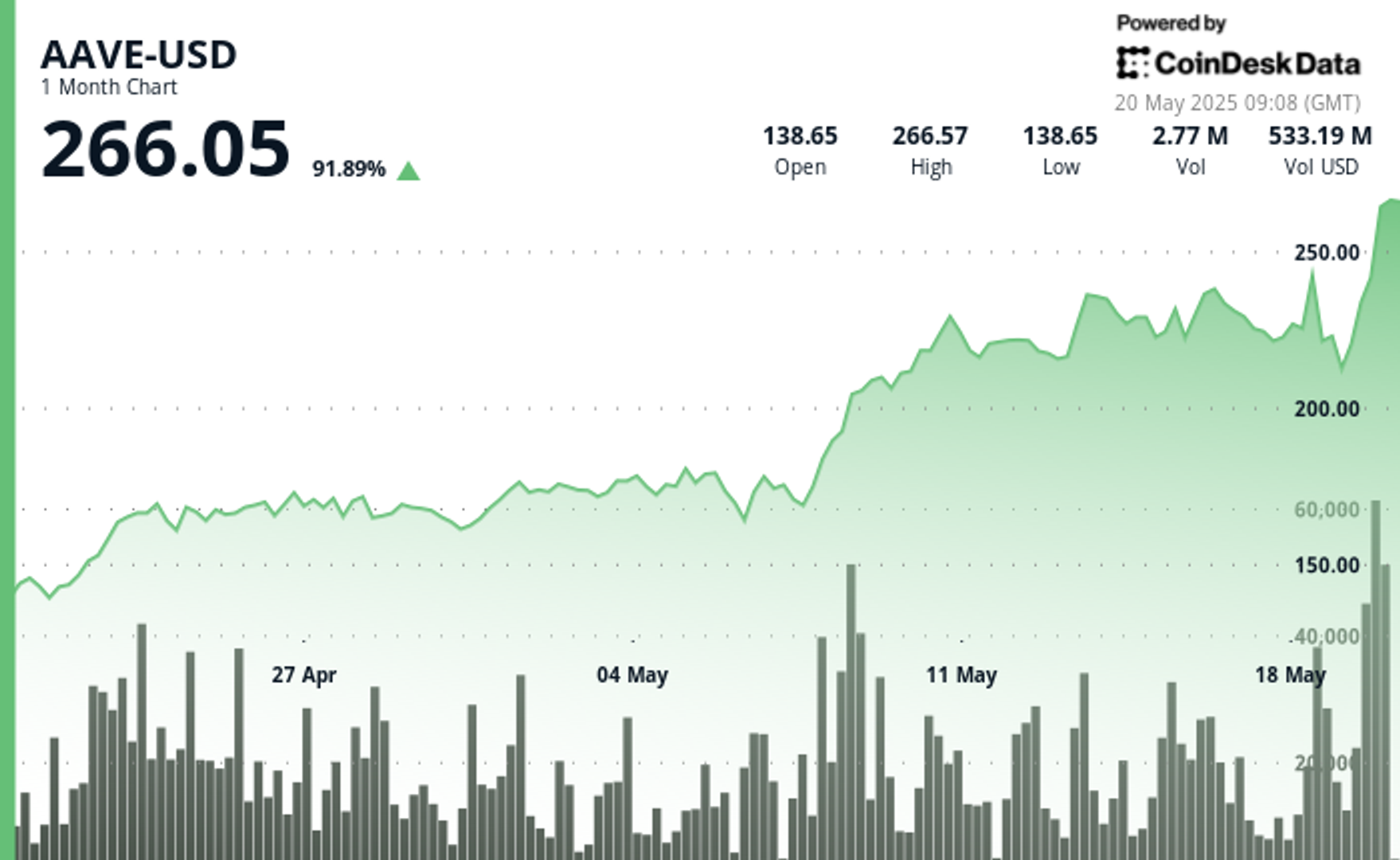
By Francisco Rodrigues (All times ET unless indicated otherwise) Bitcoin BTC surpassed Wednesday’s record to reach an all-time high of $111,875 in the early hours of Thursday, as traditional financial markets...
By Francisco Rodrigues (All times ET unless indicated otherwise)
Bitcoin BTC surpassed Wednesday’s record to reach an all-time high of $111,875 in the early hours of Thursday, as traditional financial markets contended with rising bond yields and renewed concerns over ballooning U.S. debt.
The largest cryptocurrency has gained around 3.8% in the last 24 hours while the broader CoinDesk 20 CD20 index rose 4.74%, continuing a trend of strength driven by mounting institutional demand and growing interest in crypto exposure.
The rally is unfolding against a backdrop of higher yields on U.S. and Japanese government bonds. The 10-year U.S. Treasury yield rose to 4.6%, while the 30-year topped 5%, driven by concerns over President Donald Trump’s tax bill that analysts estimate could add as much as $5 trillion to the country’s debt, according to Reuters.
In Japan, yields on 30- and 40-year government bonds also hit record highs. The country’s debt-to-GDP ratio stands at 234%, QCP Capital said, and growing scrutiny coupled with weak demand for long-dated JGBs sent yields soaring.
That matters because higher yields — and thus higher returns — on investments that are considered relatively safe tend to lower the appeal of riskier assets like stocks, not to mention cryptocurrencies. While BTC, with its history of trading as a risky asset, hasn’t shown much sign of ebbing demand, it raises the question of how long the rally can continue.
Still, traders have been building large long positions in BTC options, with the most open interest now concentrated at the $110,000, $120,000 and even $300,000 calls for contracts expiring in late June in a sign of continuing bullish conviction.
U.S.-traded spot bitcoin exchange-traded funds have also been seeing significant demand. Total net inflows hit $1.6 billion over the week, and $4.24 billion so far in May, SoSoValue data shows. The inflows, coupled with bitcoin’s price rise, have seen the ETFs’ total net assets hit a record $129 billion.
There are, however, some muted signs of bearish activity.
“The largest block flow this week continues to be ETH December call spreads, while overnight BTC butterfly positions hint that some traders are positioning for consolidation around current levels,” Wintermute OTC trader Jake O. said.
Note, he’s talking about consolidation, not declines. And traditional participants may even be too bearish. While the U.S. endured a recent credit downgrade, markets are now pricing in a 6-level cut all the way down to BBB+.
On top of that, per Jake O., a recent equities market sell-off may not be a result of repositioning given higher bond yields, but rather profit-taking after nine consecutive positive sessions. Stay alert!
What to Watch
Crypto
May 22: Bitcoin Pizza Day.
May 22: Top 220 TRUMP token holders will attend a gala dinner hosted by the U.S. president at the Trump National Golf Club in Washington.
May 30: The second round of FTX repayments starts.
May 31 (TBC): Mezo mainnet launch.
Macro
Day 3 of 3: Canadian Finance Minister François-Philippe Champagne and Bank of Canada Governor Tiff Macklem will co-host the three-day meeting of G7 finance ministers and central bank governors in Banff, Alberta.
May 22, 8 a.m.: Mexico’s National Institute of Statistics and Geography releases (final) Q1 GDP growth data.
May 22, 8:30 a.m.: Statistics Canada releases April producer price inflation data.
May 22, 8:30 a.m.: The U.S. Department of Labor releases unemployment insurance data for the week ended May 17.
May 23, 8:30 a.m.: Statistics Canada releases (Final) March retail sales data.
May 23, 10 a.m.: The U.S. Census Bureau releases April new single-family homes data.
Earnings (Estimates based on FactSet data)
May 28: NVIDIA (NVDA), post-market, $0.88
Token Events
Governance votes & calls
Arbitrum DAO is voting on launching “The Watchdog,” a 400,000-ARB bounty program to reward community sleuths for uncovering misuse of the hundreds of millions in grants, incentives and service budgets the DAO has deployed. Voting ends May 23.
Lido DAO is voting on adopting Dual Governance (LIP-28), a protocol upgrade that inserts a dynamic timelock between DAO decisions and execution so stETH holders can escrow tokens to pause proposals at 1% of TVL or fully block and “rage-quit” at 10%. Voting ends May 28.
Arbitrum DAO is voting on a constitutional AIP to upgrade Arbitrum One and Arbitrum Nova to ArbOS 40 “Callisto,” bringing them in line with Ethereum’s May 7 Pectra upgrade. The proposal schedules activation for June 17, and voting ends on May 29.
May 22: Official Trump to announce its “next Era” on the day of the dinner for its largest holders.
June 10: Ether.fi to host an analyst call followed by a Q&A session.
Unlocks
May 31: Optimism (OP) to unlock 1.89% of its circulating supply worth $24.67 million.
June 1: Sui (SUI) to unlock 1.32% of its circulating supply worth $182.58 million.
June 1: ZetaChain (ZETA) to unlock 5.34% of its circulating supply worth $11.99 million.
June 12: Ethena (ENA) to unlock 0.7% of its circulating supply worth $16.78 million.
June 12: Aptos (APT) to unlock 1.79% of its circulating supply worth $61.86 million.
Token Launches
June 1: Staking rewards for staking ERC-20 OM on MANTRA Finance end.
June 16: Advised deadline to unstake stMATIC as part of Lido on Polygon’s sunsetting process ends.
Conferences
Day 3 of 7: Dutch Blockchain Week (Amsterdam)
Day 3 of 3: Avalanche Summit London
Day 3 of 3: Seamless Middle East Fintech 2025 (Dubai)
Day 2 of 2: Crypto Expo Dubai
Day 2 of 2: Cryptoverse Conference (Warsaw)
May 27-29: Bitcoin 2025 (Las Vegas)
May 27-30: Web Summit Vancouver
May 29: Stablecon (New York)
May 29-30: Litecoin Summit 2025 (Las Vegas)
May 29-June 1: Balkans Crypto 2025 (Tirana, Albania)
June 2-7: SXSW London
June 15-17: G7 2025 Summit (Kananaskis, Alberta, Canada)
June 19-21: BTC Prague 2025
Token Talk
By Shaurya Malwa
The HYPE token is in focus after a billion-dollar bitcoin trade boosted Hyperliquid’s fundamentals.
Pseudonymous trader James Wynn opened a $1.1 billion long on BTC using 40x leverage via Hyperliquid in one of the largest on-chain DEX trades ever recorded.
The position, tied to wallet “0x507,” was entered when BTC was priced at $108K and now sits on over $40 million in unrealized profit.
Wynn booked partial profits early Thursday by closing 540 BTC (~$60 million), to net $1.5 million.
His prior exits were followed by BTC declines, so traders are watching closely.
Hyperliquid runs on its custom L1, HyperEVM, using the HyperBFT consensus (200K+ TPS) with CEX-level features like real-time order books and deep liquidity — no KYC required.
The platform’s permissionless design and lightning-fast execution are increasingly drawing capital from centralized venues to DeFi , and this trade could set a precedent for whale activity.
HYPE jumped 15% in the past 24 hours on renewed attention and usage-driven speculation.
Derivatives Positioning
Analyzing the liquidations heatmap of the BTC-USDT pair on Binance, the largest liquidations cluster around $108.5K and $106.9K with liquidations worth $143 million and $112.5 million, respectively.
Meanwhile, the options market swells post-breakout, with open interest on Deribit climbing above $34 billion, just shy of the all-time high of $35.9 billion set in December. The bulk of this positioning is centered on the 30 May expiry, which now holds over $9 billion in notional value to become a key date for potential volatility.
Bullish sentiment is clearly in control, with traders aggressively targeting upside via calls. Strikes at $100K, $120K and $150K have attracted particularly large open interest, reflecting growing conviction in a continued rally.
Put/call ratios underscore this shift in sentiment — the 24-hour volume ratio has dropped to 0.49, while the open interest ratio sits at 0.60, indicating a meaningful tilt toward bullish exposure following BTC’s move above $110K.
Near-term options activity is also picking up, with weekly and monthly contracts seeing notable inflows. Traders appear to be positioning for further momentum or short-term price swings in the wake of the breakout.
Market Movements
BTC is up 1.19% from 4 p.m. ET Wednesday at $110,690.36 (24hrs: +4.05%)
ETH is up 6.19% at $2,662.72 (24hrs: +5.23%)
CoinDesk 20 is up 3.64% at 3,348.63 (24hrs: +4.88%)
Ether CESR Composite Staking Rate is unchanged at 3.03%
BTC funding rate is at 0.03% (10.95% annualized) on Binance
DXY is up 0.25% at 99.81
Gold is down 0.26% at $3,305.6/oz
Silver is down 0.83% at $33.17/oz
Nikkei 225 closed -0.84% at 36,985.87
Hang Seng closed -1.19% at 23,544.31
FTSE is down 0.68% at 8,726.62
Euro Stoxx 50 is down 0.96% at 5,402.31
DJIA closed on Wednesday -0.91% at 41,860.44
S&P 500 closed -1.61% at 5,844.61
Nasdaq closed -1.41% at 18,872.64
S&P/TSX Composite Index closed -0.83% at 25,839.17
S&P 40 Latin America closed -1.31% at 2,597.38
U.S. 10-year Treasury rate is down 2 bps at 4.58%
E-mini S&P 500 futures are unchanged at 5,865.50
E-mini Nasdaq-100 futures are up 0.15% at 21,188.50
E-mini Dow Jones Industrial Average Index futures are down 0.17% at 41,875.00
Bitcoin Stats:
BTC Dominance: 63.90 (-0.62%)
Ethereum to bitcoin ratio: 0.02409 (3.52%)
Hashrate (seven-day moving average): 875 EH/s
Hashprice (spot): $58.24
Total Fees: 7.89 BTC / $847,124
CME Futures Open Interest: 160,740 BTC
BTC priced in gold: 33.4 oz
BTC vs gold market cap: 9.47%
Technical Analysis
Bitcoin reached a new all-time high of $111,875 this morning, breaking decisively above the previous peak just above $109,000 set in January.
With a confirmed close above that level and no sign of a swing failure pattern, the bias remains firmly tilted toward continued upside. In the near term, BTC may encounter resistance around the $112,000–$113,000 range, aligning with a trendline drawn from the prior highs in December and January.
However, last week’s consolidation above $100,000 — and the successful reclaim of the previous all-time high — suggest this area is now acting as short-term support.
A pullback below $100,000, especially into the weekly order block, would likely represent a healthy correction within the broader uptrend and could offer a compelling reentry opportunity if further downside is seen.
Crypto Equities
Strategy (MSTR): closed on Wednesday at $402.69 (-3.41%), up 1.73% at $409.67 in pre-market
Coinbase Global (COIN): closed at $258.99 (-0.91%), up 2.78% at $266.20
Galaxy Digital Holdings (GLXY): closed at C$31 (+1.57%)
MARA Holdings (MARA): closed at $15.84 (-2.16%), up 4.42% at $16.54
Riot Platforms (RIOT): closed at $8.84 (-1.01%), up 3.39% at $9.14
Core Scientific (CORZ): closed at $10.78 (-1.28%), up 1.48% at $10.94
CleanSpark (CLSK): closed at $10.11 (+4.23%), up 4.65% at $10.58
CoinShares Valkyrie Bitcoin Miners ETF (WGMI): closed at $17.75 (-1.33%)
Semler Scientific (SMLR): closed at $44.89 (+7.19%), up 6.01% at $47.59
Exodus Movement (EXOD): closed at $32.76 (-5.07%), unchanged in pre-market
ETF Flows
Spot BTC ETFs:
Daily net flow: $607.1 million
Cumulative net flows: $43.35 billion
Total BTC holdings ~ 1.19 million
Spot ETH ETFs
Daily net flow: $0.6 million
Cumulative net flows: $2.61 billion
Total ETH holdings ~ 3.49 million
Source: Farside Investors
Overnight Flows
Chart of the Day
Top 20 digital assets’ prices and volumes
Chart of the day
Bitcoin adoption